Purified drinking water, often referred to as potable water, embodies a refined aqueous solution that has undergone a rigorous process of filtration and treatment to eliminate impurities and potential health hazards. This highly sought-after elixir of hydration emerges from a series of complex mechanistic interventions implemented with the chief aim of eliminating contaminants, microorganisms, and chemical substances that may be detrimental to human consumption. The extensive purification procedure encompasses various stages such as coagulation, flocculation, sedimentation, filtration, disinfection, and pH adjustment.

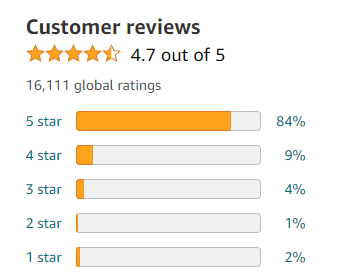
Initially, the water undergoes coagulation wherein specific chemicals are added to facilitate the formation of larger particles from smaller suspended solids. Subsequently, flocculation is employed to further aggregate these particles into even larger masses through gentle stirring or mixing. Once formed, these flocs settle down during the sedimentation phase due to gravity-induced separation. The clarified liquid then undergoes filtration using advanced techniques like activated carbon or multimedia filters which effectively trap remaining particulate matter.

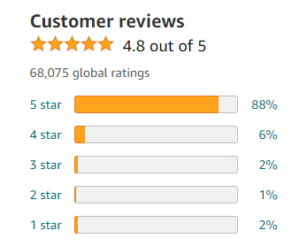
However, mere particle removal does not guarantee absolute purity; thus disinfection becomes imperative. To ensure microbial safety and prevent the transmission of waterborne diseases, various methods such as chlorination or ultraviolet irradiation are employed at this stage. Additionally, pH adjustment is carried out to optimize taste and minimize corrosion in distribution systems. Through this intricate sequence of processes designed to eliminate numerous potential hazards lurking within water sources, purified drinking water emerges as an epitome of unrivaled quality – ready for consumption by